Introduction
As with the rest of my curriculum, I’ve gone through the College Board’s Course and Exam Description for AP Biology and extracted the following Learning Objectives, Enduring Understandings, and pieces of Essential Knowledge. I’ve used this to inform the development of my on-line tutorials about biochemistry, and the instruction I’m delivering to my own students at BHS. I’d love to know what you think.
Note that I’ve put together this student learning guide that you can use to help your students stay on track and document their completion of the material in these modules (though you can also set up a qwizcards.com account and directly track student progress). I do both.
Biochemistry-related learning objectives
Source: Course and Exam Description
- LO 4.1:The student is able to explain the connection between the sequence and the subcomponents of a biological polymer and its properties. [See SP 7.1; Essential knowledge 4.A.1] (EK: 4A1)
- LO 4.2:The student is able to refine representations and models to explain how the subcomponents of a biological polymer and their sequence determine the properties of that polymer. (EK: 4A1)
- LO 4.3:The student is able to use models to predict and justify that changes in the subcomponents of a biological polymer affect the functionality of the molecule (EK: 4A1)
Biochemistry Essential Knowledge and Enduring Understandings
Source: This is a summary of material in the Course and Exam Description. Material in italics is quoted verbatim.
4.A.1: The subcomponents of biological molecules and their sequence determine the properties of that molecule.
a. Structure and function of polymers are derived from the way their monomers are assembled.
Evidence of student learning is a demonstrated understanding of each of the following:
1. In nucleic acids, biological information is encoded in sequences of nucleotide monomers. Each nucleotide has structural components: a five-carbon sugar (deoxyribose or ribose), a phosphate and a nitrogen base (adenine, thymine, guanine, cytosine or uracil). DNA and RNA differ in function and differ slightly in structure, and these structural differences account for the differing functions. [See also 1.D.1, 2.A.3, 3.A.1]
? The molecular structure of specific nucleotides is beyond the scope of the course and the AP Exam.
2. In proteins, the specific order of amino acids in a polypeptide (primary structure) interacts with the environment to determine the overall shape of the protein, which also involves secondary tertiary and quaternary structure and, thus, its function. the R group of an amino acid can be categorized by chemical properties (hydrophobic, hydrophilic and ionic), and the interactions of these R groups determine structure and function of that region of the protein. [See also 1.D.1, 2.A.3, 2.B.1]
? The molecular structure of specific amino acids is beyond the scope of the course and the AP Exam.
3. In general, lipids are nonpolar; however, phospholipids exhibit structural properties, with polar regions that interact with other polar molecules such as water, and with nonpolar regions where differences in saturation determine the structure and function of lipids. [See also 1.D.1, 2.A.3, 2. B.1]
? The molecular structure of specific lipids is beyond the scope of the course and the AP Exam.
4. Carbohydrates are composed of sugar monomers whose structures and bonding with each other by dehydration synthesis determine the properties and functions of the molecules. Illustrative examples include: cellulose versus starch.
? The molecular structure of specific carbohydrate polymers is beyond the scope of the course and the AP Exam.
b. Directionality influences structure and function of the polymer.
Evidence of student learning is a demonstrated understanding of each of the following:
- Nucleic acids have ends, de ned by the and 5′ carbons of the sugar in the nucleotide, that determine the direction in which complementary nucleotides are added during DNA synthesis and the direction in which transcription occurs (from 5′ to ). [See also 3.A.1]
- Proteins have an amino end and a carboxyl end, and consist of a linear sequence of amino acids connected by the formation of peptide bonds by dehydration synthesis between the amino and carboxyl groups of adjacent monomers.
- The nature of the bonding between carbohydrate subunits determines their relative orientation in the carbohydrate, which then determines the secondary structure of the carbohydrate.
What I Focus on in Teaching and Learning
The level of detail in what I cover with both my own students and on my on-line tutorials 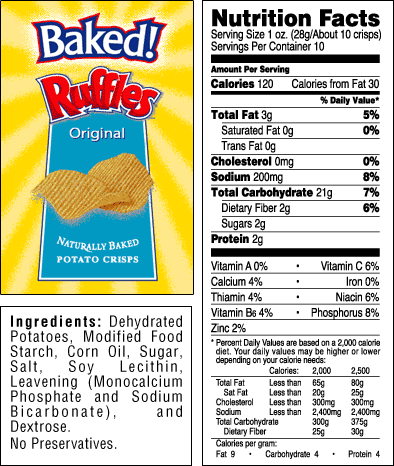 goes a bit beyond what’s in the Course Outline, and significantly beyond any question a student will have to answer on the AP exam. There’s a couple of reasons for that. First, while I believe in (and teach to) the AP test, I can’t imagine having any student who’s taken an AP Biology course being unable to interpret a food label like the one shown at left. That means teaching about the differences between a saturated and an unsaturated fat, or cis- and trans- forms of fats, and so on. Secondly, teaching what some things are called (monosaccharides vs. disaccharides, for example) doesn’t take a lot of time, and it makes talking about the many molecules that students encounter in an AP Biology course actually easier.
goes a bit beyond what’s in the Course Outline, and significantly beyond any question a student will have to answer on the AP exam. There’s a couple of reasons for that. First, while I believe in (and teach to) the AP test, I can’t imagine having any student who’s taken an AP Biology course being unable to interpret a food label like the one shown at left. That means teaching about the differences between a saturated and an unsaturated fat, or cis- and trans- forms of fats, and so on. Secondly, teaching what some things are called (monosaccharides vs. disaccharides, for example) doesn’t take a lot of time, and it makes talking about the many molecules that students encounter in an AP Biology course actually easier.
So, here’s an outline of what I teach:
- Basic ideas of biochemistry (see EK: 4.A.1):
- Monomers and Polymers
- Dehydration synthesis and hydrolysis
- Origin of monomers (preview of abiotic synthesis at the origin of life).
- Directionality
- Carbohydrates (see EK: 4.A.1.a.4)
- Monosaccharides
- Disaccharides
- Polysaccharides.
- Lipids and phospholipids (see EK: 4.A.1.a.3)
- What makes a lipid a lipid.
- Four main groups and their functions: fats and oils (energy storage and insulation), phospholipids (membranes), steroids (starting point for hormones, other molecules) and waxes (for waterproofing.
- Fatty acids can be saturated or unsaturated
- Triglycerides
- Phospholipids
- Waxes
- Steroids
- Proteins
- Diversity of function: movement, defense, signaling, catalyzing, transport, energy storage, structure)
- Monomers are amino acids.
- Levels of protein structure: primary, secondary, tertirary, quaternary
- A brief overview of nucleic acids (EK: 4.A.1.a.1). Note that in my own class, I do this through a very brief lecture. On sciencemusicvideos, this entire topic is addressed below in module 13)
Link to the Biochemistry Main Menu

