1. A gummy bear mystery
If you take a gummy bear and soak it in water for a few hours, a remarkable change will occur. As you can see, the gummy bear in water will increase in size. If you weighed it, you’d find that its mass increases up to five times. What’s going on?

2. Osmosis is water flow from hypotonic to hypertonic
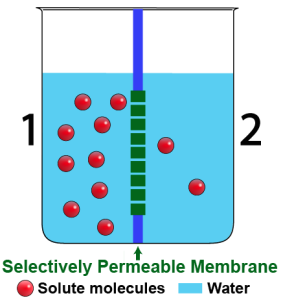
Through osmosis, water flows from higher to lower concentration.
Consider the diagram to the left. It’s a side view of a beaker that holds two solutions. The solutions are separated by a selectively permeable membrane. The membrane allows water to pass through, but not the solute, which is represented by the red spheres.
As you can see, there’s more solute on side 1 than on side 2. If there are more solute molecules, then there have to be fewer water molecules. Here’s a summary of the situation:
| Side 1 | Side 2 |
| A higher percentage of solute molecules | A lower percentage of solute molecules |
| A lower percentage of water molecules | A higher percentage of water molecules |
A somewhat outdated way to refer to a solute is as the tonic (a phrase we hold onto in phrases like “gin and tonic,” where the tonic is dissolved in the gin). Using that, we wind up with two more descriptions of the situation above:
- Side 1 is hypertonic to side 2. That means that the solution on side 1 has more solute dissolved in it than the solution on side 2.
- Side 2 is hypotonic to side 1. That means that the solution on side 2 has less solute dissolved in it than the solution on side 1.
You can remember this by focusing on the prefixes hypo and hyper.
- Hypo means under, or beneath, as in hypodermic (beneath the skin). Hypotonic means less solute.
- Hyper means too much, as in hyperactive (too active). Hypertonic means more solute.

Since water (like everything else) diffuses from higher concentration to lower concentration, we can expect that water will flow from side 2 to side 1. Another way to say that is that water will diffuse from the hypotonic side to the hypertonic side.
That’s why the green gummy bear expanded. Water flowed from the water in the cup into the gummy bear. Why? Because the water in the cup was hypotonic to the gummy bear (which was hypertonic to the water in the cup). To accommodate this inflow of water, the gummy expanded.
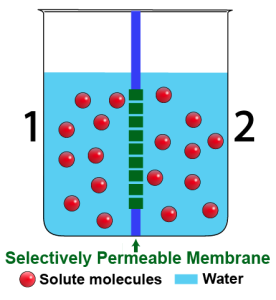
The solutions on opposite sides of a membrane can also have the same concentration of water, which is to say that they can have the same concentration of dissolved solute. The word for that is isotonic. Here’s a diagram of two solutions that are isotonic to one another.
SUMMARY:
- Osmosis is the diffusion of water.
- A hypertonic solution has relatively more dissolved solute than an adjacent solution.
- A hypotonic solution has relatively less dissolved solute than an adjacent solution.
- Isotonic solutions have equal amounts of dissolved solutes.
- Water always diffuses from hypotonic to hypertonic.
3. Osmosis Vocabulary Quiz
This quiz will help you master the following terms.
- Diffusion
- Osmosis
- Solvent
- Solute
- Solution
- Hypertonic
- Hypotonic
- Isotonic
About half of the questions ask you to describe or define a term (as opposed to just filling in the blanks. Write out or say your answer aloude, and then check it against mine. If you understand the concept, click “Got it.” Otherwise, click “need more practice.
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-Osmosis Vocabulary”]
[h]Osmosis Vocabulary
[i]
[q]The movement of a substance from higher concentration to lower concentration is [hangman].
[c]ZGlmZnVzaW9u
Cg==[Qq]
[q]The diffusion of water is [hangman].
[c]b3Ntb3Npcw==
Cg==[Qq]
[q]A mixture in which one thing is dissolved in another is a(n) [hangman].
[c]c29sdXRpb24=
Cg==[Qq]
[q]In a solution, the thing that does the dissolving is the [hangman].
[c]c29sdmVudA==[Qq]
[q]In a solution, the thing that gets dissolved is the [hangman].
[c]c29sdXRl[Qq]
[q]In lemonade (that you make from a mix), the lemonade mix is the [hangman].
[c]c29sdXRl[Qq]
[q]In lemonade (that you make from a mix), the water is the [hangman].
[c]c29sdmVudA==[Qq]
[q]Solution “A” has more dissolved solute than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwZXJ0b25pYw==[Qq]
[q]Solution “A” has less dissolved solute than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwb3Rvbmlj
Cg==[Qq]
[q]Solution “A” has the same amount of dissolved solute as solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aXNvdG9uaWM=[Qq]
[q]Solution “A” has a higher percentage of water than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwb3Rvbmlj[Qq]
[q]Solution “A” has a lower percentage of water than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwZXJ0b25pYw==[Qq]
[q]If I want to make a solution more hypertonic, I add more [hangman].
[c]c29sdXRl[Qq]
[q]If I want to make a solution more hypotonic, I add more [hangman].
[c]c29sdmVudA==[Qq]
[q]If I want to make a solution less hypertonic, I add more [hangman].
[c]c29sdmVudA==[Qq]
[q]Define diffusion. Use the word “concentration” in your definition.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]RGlmZnVzaW9uIGlzIHRoZSBtb3ZlbWVudCBvZiBtb2xlY3VsZXMgZnJvbSBoaWdoZXIgdG8gbG93ZXIgY29uY2VudHJhdGlvbi4=
Cg==[Qq]
[q]Define diffusion. Use the word “gradient” in your definition.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]RGlmZnVzaW9uIGlzIHRoZSBtb3ZlbWVudCBvZiBhIHN1YnN0YW5jZcKgZG93biBpdHMgY29uY2VudHJhdGlvbiBncmFkaWVudC4=
Cg==[Qq]
[q]Define osmosis.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]T3Ntb3NpcyBpc8KgdGhlIGRpZmZ1c2lvbiBvZiB3YXRlci4=
Cg==[Qq]
[q]Using two imaginary solutions, “A” and “B,” define hypotonic in terms of dissolved solute.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]SWYgc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGhhcyBsZXNzwqBkaXNzb2x2ZWQgc29sdXRlIHRoYW4gc29sdXRpb24gJiM4MjIwO0IsJiM4MjIxOyB0aGVuIHNvbHV0aW9uICYjODIyMDtBJiM4MjIxOyBpcyBoeXBvdG9uaWMgdG8gc29sdXRpb24gJiM4MjIwO0ImIzgyMjE7
Cg==[Qq]
[q]Using two imaginary solutions, “A” and “B,” define hypotonic in terms of water concentration.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]SWYgc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGhhcyBhIGhpZ2hlciB3YXRlciBjb25jZW50cmF0aW9uwqB0aGFuIHNvbHV0aW9uICYjODIyMDtCLCYjODIyMTsgdGhlbiBzb2x1dGlvbiAmIzgyMjA7QSYjODIyMTsgaXMgaHlwb3RvbmljwqB0byBzb2x1dGlvbiAmIzgyMjA7Qi4mIzgyMjE7
Cg==[Qq]
[q]Using two imaginary solutions, “A” and “B,” define hypertonic in terms of dissolved solute.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]SWYgc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGhhcyBtb3JlIGRpc3NvbHZlZCBzb2x1dGUgdGhhbiBzb2x1dGlvbiAmIzgyMjA7QiwmIzgyMjE7IHRoZW4gc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGlzIGh5cGVydG9uaWMgdG8gc29sdXRpb24gJiM4MjIwO0IuJiM4MjIxOw==
Cg==[Qq]
[q]Using two imaginary solutions, “A” and “B,” define hypertonic in terms of water concentration.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]SWYgc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGhhcyBhIGxvd2VywqB3YXRlciBjb25jZW50cmF0aW9uwqB0aGFuIHNvbHV0aW9uICYjODIyMDtCLCYjODIyMTsgdGhlbiBzb2x1dGlvbiAmIzgyMjA7QSYjODIyMTsgaXMgaHlwZXJ0b25pY8KgdG8gc29sdXRpb24gJiM4MjIwO0IuJiM4MjIxOw==
Cg==[Qq]
[q]Using two imaginary solutions, “A” and “B,” define isotonic in terms of dissolved solute.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]SWYgc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGhhcyB0aGUgc2FtZSBhbW91bnQgb2bCoGRpc3NvbHZlZCBzb2x1dGUgYXPCoHNvbHV0aW9uICYjODIyMDtCLCYjODIyMTsgdGhlbiBzb2x1dGlvbiAmIzgyMjA7QSYjODIyMTsgaXMgaXNvdG9uaWPCoHRvIHNvbHV0aW9uICYjODIyMDtCLiYjODIyMTs=
Cg==[Qq]
[q]Using two imaginary solutions, “A” and “B,” define isotonic in terms of water concentration.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]SWYgc29sdXRpb24gJiM4MjIwO0EmIzgyMjE7IGhhcyB0aGUgc2FtZcKgd2F0ZXIgY29uY2VudHJhdGlvbiBhc8Kgc29sdXRpb24gJiM4MjIwO0IsJiM4MjIxOyB0aGVuIHNvbHV0aW9uICYjODIyMDtBJiM4MjIxOyBpcyBpc290b25pY8KgdG8gc29sdXRpb24gJiM4MjIwO0IuJiM4MjIxOw==
Cg==[Qq]
[q]Using the terms hypotonic and hypertonic, describe how water flows.
[c]U2hvdyB0aGUgYW5zd2Vy[Qq]
[f]V2F0ZXIgYWx3YXlzIGZsb3dzIGZyb20gaHlwb3RvbmljIHRvIGh5cGVydG9uaWMu[Qq]
[x]
If you want more practice, please press the restart button below. Otherwise, follow the links below.
[restart]
[/qwiz]
Next
- Osmosis 2 (next tutorial in this series)
- Cell Membranes and Osmosis Tutorials Menu
- Osmosis! (Music Video)

